
The main goal of the first project is to understand the role of natural and sexual selection in the evolution and maintenance of genetic variation at loci coding for melanin-based colour traits by combining disciplines of behavioural ecology, genetics and population genetics. In the second project, our aim is to determine the role of nestling begging and sib-sib communication in the resolution of conflicts between parents and offspring and among siblings. We recently started a project on the evolution of language in animal communication.

The evolution, maintenance and genetics of melanin-based polymorphism
In vertebrates melanin-based coloration is often associated with variation in physiological and behavioural traits. We recently proposed that this association stems from pleiotropic effects of the genes regulating the synthesis of brown to black eumelanin. Because melanocortins act through five G-protein-coupled melanocortin receptors (MC1-5R), which have very different functions (i.e. melanin production, sexual behaviour, aggressiveness and exocrine gland activity, HPA stress response, immune function and energy homeostasis), the degree of melanin-based coloration should covary with other phenotypic traits. The finding that regulators of the melanocortin system can pleiotropically affect the expression of suites of correlated phenotypic traits has important implications with regard to the existence of behavioural syndromes. These syndromes are analogous to personality differences with some individuals being bolder or more shy and where individuals consistently differ with respect to many traits across a range of situations and contexts. We proposed that behavioural syndromes might evolve by pleiotropic effects, whereby different strategies are signalled by melanin-based coloration. Indeed, darker eumelanic individuals are usually more aggressive, sexually active, immunocompetent and resistant to stress, traits that can be useful for bold individuals being explorative and taking more risks. Less eumelanic individuals might adopt the opposite strategy to derive the same fitness as dark conspecifics.
The aim of our research is to:
- determine the adaptive function of alternative melanin-based colour traits,
- identify how ecological, social and physiological factors jointly influence and maintain inter-individual variation in melanin-based coloration and the other associated phenotypic traits,
- examine whether the level of melanocortins in different tissues correlate with melanin-based coloration,
- identify the mechanism underlying a difference in melanocortin levels between differently coloured individuals (i.e. genetic polymorphism, expression of the POMC mRNA or post-translational modification of the POMC prohormone), and
- manipulate melanocortin level to demonstrate that these hormones are responsible for the production of melanin-based coloration and its covariation with other phenotypic traits as observed in natural situations.
Aim, method and originality of the project
To study the evolution, maintenance and genetics of melanin-based coloration, we mainly work with three species: the barn owl, tawny owl and kestrel. These birds display polymorphism in melanin-based coloration which signal different individual attributes. These three species of birds vary in a number of life history traits and ecological aspects, and are therefore suited to perform a thorough study on melanin-based coloration. More specifically, we investigate the following key issues:
- The signalling function of melanin-based coloration. To this end, we perform experiments in the field with wild animals, and analyse a long-term database (20 years of data).
- Genetics of melanin-based coloration. This part is done in the laboratory using molecular techniques.
This project is original, since the interest in melanin-based colour traits and genetic colour polymorphism has recently grown. Furthermore, we recently proposed a new genetic mechanism to explain why melanin-based colour traits are frequently associated with several individual attributes.
Key papers
Ducrest, A.-L., Keller, L. & Roulin, A. In press. There is more to colour than what you see: the pleiotropic effects of the melanocortin system. Trends in Ecology and Evolution.
Roulin, A. & Altwegg, R. 2007. Breeding rate is associated with pheomelanism in male and with eumelanism in female barn owls. Behavioral Ecology 18: 563-570.
Roulin, A. 2004. Proximate basis of the covariation between a melanin-based female ornament and offspring quality. Oecologia 140, 668-675.
Roulin, A. 2004. The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biological Reviews 79, 815-848.
Roulin, A. & Dijkstra, C. 2003. Genetic and environmental components of variation in eumelanin and phaeomelanin sex-traits in the barn owl. Heredity 90, 359-364.

Parent-offspring conflict, sibling negotiation and language evolution
When siblings strongly differ in need, in the absence of parents they signal to each other their willingness to compete for non-divisible food at their parent's return. A needy individual signals to its siblings that it will vigorously contest the impending food resources in order to deter siblings from competing when parents return to the nest, thus ensuring that it will be fed without having to beg too intensely. In contrast, since less needy siblings have little chance of being fed, they may expect little reward from investment in sibling competition. They should refrain from signalling to siblings, therefore indicating that they will retreat from sibling competition. This would allow them to avoid wasting energy in negotiation, competitive behaviour and begging. In sum, sibling negotiation should allow nestlings to optimally invest effort in competitive begging mainly when food resources are non-divisible. The sibling negotiation hypothesis has been demonstrated in the barn owl and recently in spotless starling (Bulmer et al. 2007 Behav. Ecol. 19, 279-284).
Aim, method and originality of the project
In this project, we study the adaptive value of sibling interactions by using an experimental approach with wild barn owl broods. This species is particularly suited because nestlings vocalise all night long even when parents are not at the nest. These vocalisations are directed to siblings and signal food need.
- Currently, we have five major aims:
- Importance of individual recognition in sib-sib communication, since calls of each individual is distinctive
- Importance of begging behaviour and sibling negotiation on within-brood food allocation
- Dynamics over time of begging behaviour and sibling negotiation
- Role of punishment to reinforce the honesty of nestling calls as a honest signal of need
- Evolution of nest-specific signals of need. This project encompasses also the evolution of proto-language
This project is original because the importance of sib-sib communication in the resolution of conflicts between parents and offspring and among siblings has not been considered in detail. This system also allows us to investigate original questions linked to individual recognition, punishment and the evolution of language.
Key papers
Roulin, A., Kölliker, M. & Richner, H. 2000. Barn owl (Tyto alba) siblings vocally negotiate resources. Proceedings of the Royal Society of London B267, 459-463.
Johnstone, R. A. & Roulin, A. 2003. Sibling negotiation. Behavioral Ecology 14, 780-786.
Roulin, A. 2002. The sibling negotiation hypothesis. In The evolution of begging: competition, cooperation and communication (eds. Wright J. & Leonard M.), Dordrecht, Kluwer Academic Press, pp. 107-127.
Roulin, A. 2004. Effects of hatching asynchrony on sibling negotiation, begging, jostling for position and within-brood food allocation in the barn owl Tyto alba. Evolutionary Ecology Research 6, 1083-1098.
Melanin-Based Colour Polymorphism - Melanocortin System
RESEARCH
Our general interest
We are interested in the evolution, maintenance and adaptive function of alternative behavioural, physiological and life history strategies that coexist in a given population. We study this aspect at three different temporal and special scales: (1) Within population. We investigate the causes of consequences of alternative strategies prevailing in the same population. To this end we record the performance of individuals that compete over the same pool of resources (e.g. food, mate, territories). (2) Between populations. We examine the factors that explain why populations do not evolve the same physiological, morphological, behavioural and life history traits. (3) Between species. The key issue here is to understand why species differ phenotypically but also why different species often evolved similar strategies.
Colour polymorphism is a model system to study genetically inherited strategies as well as the adaptive function, maintenance and evolution of polymorphism

Example of a colour polymorphic species, the tawny owl. Birds from the same population can display one of several distinct colour morphs (for studies on the tawny owl see for instance Piault R, Gasparini J, Bize P, Jenni-Eiermann S & Roulin A. 2009. Pheomelanin-based coloration and the ability to cope with variation in food supply and parasitism. American Naturalist 174, 548-556; Gasparini J, Bize P, Piault R, Kazumasa W, Blount J & Roulin A. 2009. Strength and cost of mounting an immune response are associated with a heritable melanin-based color trait in female tawny owls. Journal of Animal Ecology 78, 608-616; Gasparini, J, Piault R, Bize P & Roulin A. 2009. Synergistic and antagonistic interactions between different branches of the immune system is related to melanin-based coloration in nestling barn owls. Journal of Evolutionary Biology 22, 2348-2353; Roulin A, Ducret B, Ravussin P-A & Altwegg R. 2003. Female plumage coloration covaries with reproductive strategies in the tawny owl. Journal of Avian Biology 34, 393-401)
Environmentally-mediated strategies vs. genetically-inherited strategies
Behaviour is typically very sensitive to environmental factors such as food or parasites, whereas morphology and physiology can have a strong underlying genetic basis. To study a large diversity of strategies, an appropriate approach is to study behaviour and traits that are known to be under tight genetic control. This is why we study two model systems, the first one being behavioural interactions taking place between family members. Offspring are in conflict with their parents over how much parents should invest in parental care; siblings are in conflict over how parental resources should be shared; parents are in conflict over much each parent should assume parental care. To resolve these conflicts parents and offspring have evolved a battery of behavioural strategies. The second model system is melanin-based colour polymorphism. Here our interest is driven by the very strong genetic determinism of expressing alternative colour morphs and by the fact that different colour morphs are often associated with a number of morphological, behavioural, physiological and life history traits that are themselves sensitive to environmental factors (in contrast to colour morphs themselves). These two model systems allow us to study strategies that are purely behavioural (i.e. weakly sensitive to genetic factors) and that are under very tight genetic control (i.e. an individual cannot choose its strategy that is genetically programmed).

Variation in the degree of melanin-based coloration in eastern Hermann’s tortoises (see Mafli A, Wakamatsu K & Roulin A. 2011. Melanin-based colouration predicts aggressiveness and boldness in captive Eastern Hermann’s tortoises. Animal Behaviour 81, 859-863). Photo Alia Mafli.
The barn owl
I am working with the barn owl long before I started the university. As a teenager I was an enthusiastic ornithologist who dedicated his free time with great friends to ring all kind of birds (from small passerines to the Golden eagle). This experience allowed me to learn how to work with birds and was key to select a “model organism” with which I would dedicate not only my free time but my academic research. Among all animals I could see and handle, the barn owl was the definite species to work with. Not because I love it more than other species but because it is highly interesting, at least from my point of view. Arguments:
- Cosmopolitan: it is one of the 8 bird species to be distributed worldwide. Almost everywhere you go, you will find a barn owl.
- Colour polymorphism: individuals vary with respect to plumage coloration from dark reddish brown to white and from immaculate to heavily marked with large black spots. This variation is observed at all spatial scales (between continents, countries, populations, within populations and within families). Not two barn owls are similar. I could myself recognize barn owls individually among hundreds.
- Reproduction: the barn owl has an extraordinary reproductive potential. This bird can produce two to three annual broods, up to 11 chicks at fledging and in some countries it can breed all year long.
- Hatching asynchrony: because females start to incubate their eggs as soon as the first egg is laid, the oldest nestling is sometimes born one month before its youngest sibling.
- Population dynamics: because the barn owl is very sensitive to environmental conditions, at least much more than closely related owl species, we can observe huge variation in population size between years.
- Physiology: the barn owl is a model system to study hearing capacity, vision and the ability to fly silently.
- Peaceful sib-sib interactions: young siblings vocally negotiate priority of access to food resources and they altruistically feed their younger siblings.
- Stealing behaviour: young barn owls not only share food peacefully within the family but can also be egoistic. For instance, siblings can steal food from each other.
- Food stores: because nestlings spread their meals over 24 hours, we can frequently observe nestlings eating a vole during daylight hours. To do so, nestlings store food at night to be consumed during the day.
And so on. The barn owl shows so many interesting traits that it can be endlessly studied. Not only this but it allows us to bring together many fascinating aspects of its daily life in a single study framework.

A family of barn owls. Photo A. Roulin
Why to study wild barn owl and not laboratory mice or snails?
People (not only the general public but also researchers including evolutionary ecologists) formulate the following remark: “you work with owls rather than with mice or snails because you love owls. You are not a serious scientist just somebody who was lucky enough to become a scientists and do what you like (i.e. observe owls). Your research is just a curiosity, not something that deserves the same kind of academic respect as those working in the lab. Even other evolutionary ecologists had the intelligence to chose a smaller organism that require less effort”. There are three responses to these types of remarks.
Cost/benefit ratio analysis.
It is true that studying barn owl is very costly in terms of time invested (you have to work at day and night and to travel thousands of kilometres each year to visit all breeding pairs). However, this species is so interesting that the benefits of our studies are so high that the cost/benefit ratio is in the end in our favour.
Complementarity.
Studies on laboratory mice are fascinating but wild barn owls are not inbred lines and they live in the wild. This allows us to study an organism in the environment to which it has been selected.
New ideas.
Studying an organism that very few researchers “use” allows us to come with new stimulating ideas.

Study area where we study a free-living population of barn owls in Switzerland (left) and a barn owl nest box fixed to a barn (right). Photos N. Rattenborg and M. Scriba.
Melanin-based colour polymorphism
Why is the study of melanin-based coloration so interesting?
The key issue is that melanin production is under strong genetic control and melanogenesis is very conserved in all vertebrates. As a consequence, we can identify genes that are responsible for the expression of dark reddish or white plumage and if our aim is to draw conclusions out of our studies that could be applicable to a large number of organisms (including human), studies performed in the barn owl (or any other non-model organism) are as worth as those performed in laboratory mice.

Example of colour polymorphism in the asp viper (Vipera aspis; A: concolor; B: lined; C: blotched, and D: melanistic). Photo S. Dubey

Pair of Eleonora’s falcons, dark and pale morph. (Gangoso L, Grande JM, Ducrest A-L, Figuerola J, Bortolotti G, José A & Roulin A. 2011. Mc1R-dependent melanin-based colour polymorphism is associated with cell-mediated response in the Eleonora’s falcon. Journal of Evolutionary Biology 24, 2055-2063). Photo L. Gangoso.
The move towards more genetics
During about 15 years we worked at the phenotypic level. Our main aim was to determine whether differently coloured owls differ with respect to other traits including resistance to parasites, oxidative stress, a rise in blood circulating corticosterone and lack of food. These studies indeed showed that darker owls behave differently, have a different physiology and life history characteristics than lighter coloured conspecifics. This was a necessary step but it is now time to move on towards other frontiers. Before explaining what are our current aims, I sum up our current knowledge about the adaptive function of melanin-based coloration and how variation in coloration could be maintained within populations.

A barn owl parent feeding its offspring in the Middle East. Photo A. Ezer
Quantitative genetics of melanin-based coloration
This is probably the most important aspect to study when we are interested in the adaptive function of any phenotype. The key issue is to determine why an individual exhibits a different phenotype than its sibling or why it resembles an unrelated conspecific. Is a barn owl reddish-brown because it consumed different prey species than a white barn owl or is it because it possesses different genes. To answer this question we performed the so-called “cross-fostering” experiments where we swapped eggs or hatchlings between randomly chosen pairs of nests. In this way, nestlings were raised by randomly chosen parents (that do not resemble the biological parents). Therefore, if the cross-fostered nestlings develop a plumage that is similarly coloured as the plumage of their biological parents or of their siblings raised in another nest we can conclude that some genes are responsible for producing colour traits, since these individuals share genes but not the environment. In contrast, if the cross-fostered nestlings resemble their foster parents or their unrelated nestmates we can conclude that the environment has a non-negligible effect on the expression of plumage coloration, since these unrelated individuals do not share genes but the environment where they live. Our studies demonstrated that related individuals resemble each other with respect to coloration because they have genes in common but not because they live in the same environment. Regardless of the food an individual consumes or of its physical condition, it will express the colour morph that is inherited from its biological parents.
An experiment showed that when rearing conditions are extremely stressful, nestlings produce lighter coloured feathers. Indeed, a rise in blood circulating corticosterone levels during a couple of days reduces the production of pheomelanin (we could not test whether this effect also applied to eumelanin). Even if we can show that corticosterone can affect the expression of melanin-based coloration, our observations nevertheless indicate that inter-individual variation in reddish coloration is not due to the fact that these individuals experience different levels of stress. It implies that even coloration can be sensitive to environmental factors in the barn owl, the genetic component is nevertheless much more pronounced that the environmental component of colour variation.
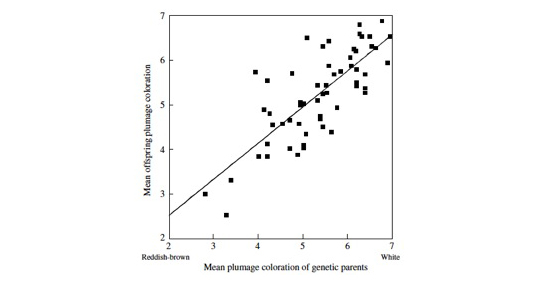
Parent-offspring resemblance in plumage coloration. From “Roulin A & Dijkstra C. 2003. Genetic and environmental components of variation in eumelanin and phaeomelanin sex-traits in the barn owl. Heredity 90, 359-364.”

Barn owl siblings. Photo A. Roulin.
For more information see
- Roulin A, Richner H & Ducrest A-L. 1998. Genetic, environmental and condition-dependent effects on female and male plumage ornamentation. Evolution 52, 1451-1460.
- Roulin A & Dijkstra C. 2003. Genetic and environmental components of variation in eumelanin and phaeomelanin sex-traits in the barn owl. Heredity 90, 359-364.
- Roulin A. 2004. Covariation between plumage colour polymorphism and diet in the barn owl Tyto alba. Ibis 146, 509-517.
- Roulin A, Altwegg R, Jensen H, Steinsland I & Schaub M. 2010. Sex-dependent selection on an autosomal melanic female ornament promotes the evolution of sex ratio bias. Ecology Letters 13, 616-626.
- Roulin A, Almasi B, Rossi-Pedruzzi A, Ducrest A-L, Wakamatsu K, Miksik I, Blount JD, Jenni-Eiermann S & Jenni L. 2008. Corticosterone mediates the condition-dependent component of melanin-based coloration. Animal Behaviour 75, 1351-1358.
Melanin pigments
An important aspect to understand the adaptive function of colour polymorphism is to determine the identity of the melanin pigments used to produce various colour traits. Thanks the help of Kazumasa Wakamatsu from the University of Fujita we could show that a reddish plumage mainly consists of pheomelanin (i.e. reddish) pigments and a little bit of eumelanin (i.e. black) pigments. These pigments are exactly the same as the ones found in human.

Alpine swifts displaying an immaculate breast or black streaks. From Bize P, Gasparini J, Klopfenstein A, Altwegg R & Roulin A. 2006. Melanin-based coloration is a non-directionally selected sex-specific signal of offspring development in the Alpine swift. Evolution 60, 2370-2380. Photo P. Bize.
For more information see
Roulin A, Almasi B, Rossi-Pedruzzi A, Ducrest A-L, Wakamatsu K, Miksik I, Blount JD, Jenni-Eiermann S & Jenni L. 2008. Corticosterone mediates the condition-dependent component of melanin-based coloration. Animal Behaviour 75, 1351-1358.
Sexual dimorphism in plumage traits
Barn owls are sexually dimorphic with respect to plumage traits. Females are on average darker reddish than males but also display on average more and larger black spots on the ventral body side than males. Interestingly, males and females can express any colourful phenotypes, i.e. we can find male-like females displaying a light reddish plumage marked with a few and small black spots or female-like males displaying a dark reddish plumage maculated with many large spots. This system therefore offers a nice opportunity to investigate whether a female-like plumage is costly to males and also whether it is more costly for females to display a male-like plumage compared to males displaying a female-like plumage.

A pair of barn owls for which the female (left) and male (right) mainly differ with respect to reddish pheomelanin-based coloration. Photo J. Jeanmonod.
For more information see
Roulin A, Altwegg R, Jensen H, Steinsland I & Schaub M. 2010. Sex-dependent selection on an autosomal melanic female ornament promotes the evolution of sex ratio bias. Ecology Letters 13, 616-626.
Age-related change in plumage traits
In many animals and birds in particular, juveniles exhibit that is not yet the one showed by adults. In most species, at the first moult yearlings get the typical plumage displayed by adults. For researchers this is a problem because when we visit nests we cannot yet determine what will be the adult coloration of each individual nestling. In contrast to most birds, young barn owls already show their adult plumage. This is very convenient because we can then test whether aspects of nestling quality (such as resistance to parasites) are correlated with melanin-based coloration measured in the nestlings themselves, in their biological mother and/or father but also in their foster mother and/or father in the case hatchlings had been previously cross-fostered between randomly chosen nests.
In collaboration with Amélie Dreiss who is able to discover everything that is possible to discover in a dataset, we found that both males and females become lighter reddish between the first and second year of life; at subsequent age birds still continue to become a little bit lighter coloured, but this change of coloration is weak. Perhaps more interestingly, we found that only males lose black spots with age, whereas only females produce larger spots between the first and second year of age. Interestingly, the extent of plumage change between two years is correlated with a change in reproductive success. Females that display much larger black spots between two years are able to reproduce much earlier in the season and they also produce larger eggs. Males that lose more black spots between two years produce more offspring.
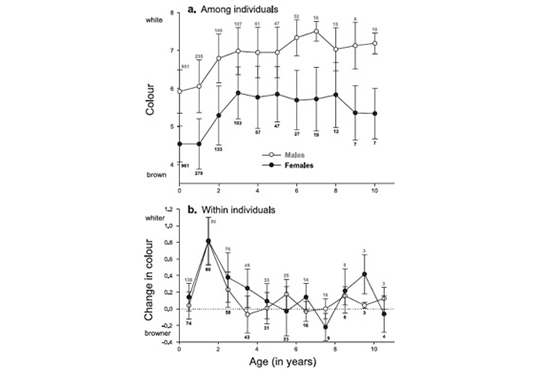

For more information see
Roulin A. 1999. Delayed maturation of plumage coloration and plumage spottiness in the barn owl Tyto alba. Journal of Ornithology 140, 193-197.
Dreiss AN & Roulin A. 2010. Age-related change in melanin-based coloration: females that become more female-like and males more male-like with age perform better in barn owls (Tyto alba). Biological Journal of the Linnean Society 101, 689-704.
Genetic correlates of melanin-based coloration
When we observe such a huge variation in plumage coloration both within and between nests, the first question that comes into our mind is whether dark reddish individuals differ in some respect from white individuals? And are birds heavily marked with black spots different from immaculate conspecifics? Intense field work since 1996 has revealed striking differences between phenotypes. Our experimental design to demonstrate a genetic link between plumage traits and other phenotypic attributes was relatively simple: we swapped eggs or hatchlings between nests so that nestlings were reared by randomly chosen foster parents. When nestlings were 1 to 2 months older we measured them in several different ways (e.g. body size, number of ectoparasites, antibody production, etc.) and correlated these measures with plumage traits measured in the nestling themselves and in both their foster and biological parents. Before reporting a summary of these results, it is worth mentioning a fact that was consistently found during all these years: most phenotypic traits measured in the cross-fostered nestlings were correlated with the size of black feather spots measured in their biological mother. More rarely, these phenotypes were correlated with spot size measured in the nestling themselves or in their biological father. We almost never observed a single correlation between nestling phenotype and plumage traits measured in the foster parents.

Measuring the uropygial gland, wing, tarsus and bill length, as well as counting and measuring the number of black spots on the flank and breast. Photo M. Charter.
Phenotypic traits associated with the size of eumelanic black spots

We can the black spots on the breast. Photo P.-A. Ravussin.
Host-parasite interactions
- Offspring born from larger-spotted females produce more antibodies specifically directed against a vaccine (Roulin A, Jungi TW, Pfister H & Dijkstra C. 2000. Female barn owls (Tyto alba) advertise good genes. Proceedings of the Royal Society of London B267, 937-941).
- Offspring born from larger-spotted females have fewer ectoparasites (fly Carnus hemapterus) (Roulin A, Riols C, Dijkstra C & Ducrest A-L. 2001. Female plumage spottiness and parasite resistance in the barn owl (Tyto alba). Behavioral Ecology 12, 103-110).
- …and these ectoparasites are less fecund suggesting that the size of maternal spots signal the ability to reduce parasite fitness (Roulin A, Riols C, Dijkstra C & Ducrest A-L. 2001. Female plumage spottiness and parasite resistance in the barn owl (Tyto alba). Behavioral Ecology 12, 103-110).
- Larger spotted females have a smaller bursa of Fabricius, an organ that produces B immune cells (Roulin A, Riols C, Dijkstra C & Ducrest A-L. 2001. Female plumage spottiness and parasite resistance in the barn owl (Tyto alba). Behavioral Ecology 12, 103-110).
Developmental homeostasis
- Offspring born from larger-spotted females produce a more symmetric body, as measured by bilateral symmetry of wing feathers (Roulin A, Ducrest A-L, Balloux F, Dijkstra C & Riols C. 2003. A female melanin-ornament signals offspring fluctuating asymmetry in the barn owl. Proceedings of the Royal Society of London B270, 167-171)
- Adult males show a higher bilateral symmetry of wing feathers (Roulin A, Ducrest A-L, Balloux F, Dijkstra C & Riols C. 2003. A female melanin-ornament signals offspring fluctuating asymmetry in the barn owl. Proceedings of the Royal Society of London B270, 167-171).
Regulation of corticosterone
The above finding about developmental homeostasis could be explained by the better regulation of corticosterone by larger-spotted birds.
- Offspring born from larger-spotted females mount a lower corticosterone response when we handle them (Almasi B, Jenni L, Jenni-Eiermann S & Roulin A. 2010. Regulation of stress-response is heritable and functionally linked to melanin-based coloration. Journal of Evolutionary Biology 23, 987-996).
- Offspring born from larger-spotted females are better able to reduce the level of an experimentally-induced elevation of blood circulating corticosterone (Almasi B, Jenni L, Jenni-Eiermann S & Roulin A. 2010. Regulation of stress-response is heritable and functionally linked to melanin-based coloration. Journal of Evolutionary Biology 23, 987-996).
- Offspring born from larger-spotted females suffer less from an experimental elevation of corticosterone as measured by fledging body mass (Almasi B, Roulin A, Korner-Nievergelt F, Jenni-Eiermann S & Jenni L. 2012. Coloration signals the ability to cope with elevated stress hormones: effects of corticosterone on growth of barn owls is associated with melanism. Journal of Evolutionary Biology 25, 1189-1199).
- Nestlings displaying larger black spots breath at a lower rate when we handle them suggesting that they are less stressed (Van den Brink V, Dolivo V, Falourd X, Dreiss AN & Roulin A. 2012. Melanic color-dependent anti-predator behavior strategies in barn owl nestlings. Behavioral Ecology 23, 473-480).
- Daily variation in baseline corticosterone levels in nestlings covaries with spot diameter displayed by their biological parents. When their mother displayed larger spots, nestlings had lower corticosterone levels in the morning and higher levels in the evening, whereas the opposite pattern was found with the size of paternal spots (Roulin A, Almasi B & Jenni L. 2010. Temporal variation in glucocorticoid levels during the resting phase is associated in opposite way with maternal and paternal melanic coloration. Journal of Evolutionary Biology 23, 2046-2053).
Anti-predator behaviour
- Larger-spotted nestlings try to scare predators to a larger degree than small spotted nestlings (Van den Brink V, Dolivo V, Falourd X, Dreiss AN & Roulin A. 2012. Melanic color-dependent anti-predator behavior strategies in barn owl nestlings. Behavioral Ecology 23, 473-480).
- Offspring born from larger-spotted mothers feign death for longer periods of time in the presence of a predator (Van den Brink V, Dolivo V, Falourd X, Dreiss AN & Roulin A. 2012. Melanic color-dependent anti-predator behavior strategies in barn owl nestlings. Behavioral Ecology 23, 473-480).
- Offspring sired by smaller-spotted fathers are more aggressive and agitated when we handle them (Van den Brink V, Dolivo V, Falourd X, Dreiss AN & Roulin A. 2012. Melanic color-dependent anti-predator behavior strategies in barn owl nestlings. Behavioral Ecology 23, 473-480).
Resistance to oxidative stress
- Nestlings displaying larger black spots were more resistant against oxidative stress (Roulin A., Almasi B, Stier K & Jenni L. 2011. Eumelanin- and pheomelanin-based color advertises resistance to oxidative stress in opposite ways. Journal of Evolutionary Biology 24, 2241-2247).
Calcium physiology
- Calcium concentration in the humerus is higher in adults displaying larger black spots (Roulin A, Dauwe T, Blust R, Eens M & Beaud M. 2006. A link between eumelanism and calcium physiology in the barn owl. Naturwissenschaften 93, 426-430).
Feather quality and preening behaviour
- Larger spotted nestlings spend less time preening their body feathers (Roulin A. 2007. Melanin pigmentation negatively correlates with plumage preening effort in barn owls. Functional Ecology 21, 264-271).
- The uropygial gland of larger-spotted adults is less heavy than the gland of smaller-spotted adults (Roulin A. 2007. Melanin pigmentation negatively correlates with plumage preening effort in barn owls. Functional Ecology 21, 264-271).
- The vane of wing and tail feathers is heavier in larger-spotted adults suggesting that their feathers are of higher quality (Roulin A. 2007. Melanin pigmentation negatively correlates with plumage preening effort in barn owls. Functional Ecology 21, 264-271).
- The calamus of wing feathers is heavier in larger-spotted adults suggesting that their feathers are of higher quality (Roulin A. 2007. Melanin pigmentation negatively correlates with plumage preening effort in barn owls. Functional Ecology 21, 264-271).
Age at maturity
- Larger-spotted females breed for the first time at an earlier age than small-spotted females (Roulin A & Altwegg R. 2007. Breeding rate is associated with pheomelanism in male and with eumelanism in female barn owls. Behavioral Ecology 18, 563-570).
Parental care
- Larger-spotted breeding males feed their brood at a higher rate than smaller-spotted males (Almasi B, Roulin A, Jenni-Eiermann S & Jenni L. 2008. Parental investment and its sensitivity to corticosterone is linked to melanin-based coloration in barn owls. Hormones and Behavior 54, 217-223).
- Males mated to females with reduced plumage spottiness fed their brood at a lower rate and as a consequence their offspring grew at a lower rate (Roulin A. 1999. Nonrandom pairing by male barn owls Tyto alba with respect to a female plumage trait. Behavioral Ecology 10, 688-695).
Reproduction
- Females that became larger-spotted from one year to the next advanced breeding date whereas those that become smaller-spotted delayed breeding. Furthermore, females produced larger eggs after their spots became larger (Dreiss AN & Roulin A. 2010. Age-related change in melanin-based coloration: females that become more female-like and males more male-like with age perform better in barn owls (Tyto alba). Biological Journal of the Linnean Society 101, 689-704).
Body maintenance during reproduction
- Larger-spotted breeding females are heavier than smaller-spotted females but only in the afternoon, not in the morning (Roulin A. 2009. Covariation between eumelanic pigmentation and body mass only under specific conditions. Narturwissenschaften 96, 375-382).
Appetite
- When offered food ad libitum nestlings displaying larger black spots consumed less food than smaller-spotted nestlings. Thus, the size of black spots is negatively correlated with appetite (Dreiss A, Henry I, Ruppli C, Almasi B & Roulin A. 2010. Darker eumelanic barn owls better withstand food depletion through resistance to food deprivation and low appetite. Oecologia 164, 65-71).
Resistance to food deprivation
- Large-spotted nestlings food-deprived during 24h lost less body mass compared to small-spotted nestlings (Dreiss A, Henry I, Ruppli C, Almasi B & Roulin A. 2010. Darker eumelanic barn owls better withstand food depletion through resistance to food deprivation and low appetite. Oecologia 164, 65-71).
Survival
- Offspring sex ratio is biased in relation to spot size displayed by both parents: mothers displaying small spots (i.e. male-like females) produce more sons, whereas fathers displaying large spots (i.e. female-like males) produce more daughters. This is valid only for the first-born offspring (Roulin A, Altwegg R, Jensen H, Steinsland I & Schaub M. 2010. Sex-dependent selection on an autosomal melanic female ornament promotes the evolution of sex ratio bias. Ecology Letters 13, 616-626).
- Larger-spotted females have a much higher survival in the first-year of life than small-spotted females. The opposite trend is observed in males (Roulin A & Altwegg R. 2007. Breeding rate is associated with pheomelanism in male and with eumelanism in female barn owls. Behavioral Ecology 18, 563-570 AND Roulin A, Altwegg R, Jensen H, Steinsland I & Schaub M. 2010. Sex-dependent selection on an autosomal melanic female ornament promotes the evolution of sex ratio bias. Ecology Letters 13, 616-626).
- Interestingly, females show a higher first-year survival when their father display large than small black spots (i.e. when their father is female-like), whereas males show a higher higher first-year survival when their mother display small spots (i.e. when their mother is male-like) (Roulin A, Altwegg R, Jensen H, Steinsland I & Schaub M. 2010. Sex-dependent selection on an autosomal melanic female ornament promotes the evolution of sex ratio bias. Ecology Letters 13, 616-626).
CONCLUSION REGARDING THE ADAPTIVE VALUE OF BLACK EUMELANIC SPOTS
Three key messages can be derived from the above review of studies:
- The size of black spots is positively genetically correlated with female quality.
- In our study area the benefit of displaying larger black spots in females is higher than the costs incurred by males who display large spots.
- Males who display a female-like plumage (i.e. large spots) and females a male-like plumage (i.e. small spots) have evolved strategies to do the best of a bad job.
As mentioned above barn owls do not only vary with respect to the size of black eumelanic spots but also in the degree of reddishness. As we will see below the adaptive function of these two traits is fundamentally different, even if these traits are genetically correlated within individuals (by the way to a larger extent in males than females), i.e. darker reddish birds commonly display larger black spots than light reddish individuals. If the size of black spots reveal female genetic quality, reddish coloration seems to be associated with predator-prey interactions and social behaviours as explained here:
Phenotypic traits associated with the reddish pheomelanin-based coloration

Barn owls vary in the degree of reddishness. Here is a pair of barn owls with an extremely white male and extremely dark reddish female. Females are on average darker reddish than males. Photo Pierre Alain-Ravussin.
Reproduction
- In juveniles darker reddish females have larger ovary than light coloured females (Roulin A. 2009. Melanin-based coloration covaries with ovary size in an age-specific manner in the barn owl. Naturwissenschaften 96, 1177-1184).
Parental care
- Darker reddish males feed their brood at a higher rate than light reddish males (Roulin A, Riols C, Dijkstra C & Ducrest A-L. 2001. Female- and male-specific signals of quality in the barn owl. Journal of Evolutionary Biology 14, 255-267).
- Darker reddish birds have a heavier heart than light reddish males (Roulin A, Riols C, Dijkstra C & Ducrest A-L. 2001. Female- and male-specific signals of quality in the barn owl. Journal of Evolutionary Biology 14, 255-267).
Diet
- Darker reddish owls eat more voles than light reddish individuals who consume more mice (Muridae). This result applies to both a Swiss and Israeli populations (Roulin A. 2004. Covariation between plumage colour polymorphism and diet in the barn owl Tyto alba. Ibis 146, 509-517; Roulin A. 2004. Covariation between plumage colour polymorphism and diet in the barn owl Tyto alba. Ibis 146, 509-517).
Habitat
- Darker reddish females breed more often in open habitats and whitish females near forests. This was observed both in Switzerland and Israel (Dreiss AN, Antoniazza S, Burri R, Fumagalli L, Sonnay C, Frey C, Goudet J & Roulin A. 2012. Local adaptation and matching habitat choice in female barn owls with respect to melanic coloration. Journal of Evolutionary Biology 25, 103-114; Charter M, Peleg O, Leshem Y & Roulin A. 2012. Similar patterns of local barn owl adaptation in the Middle East and Europe with respect to melanic coloration. Biological Journal of the Linnean Society 106, 447-454).
Local adaptation
- Whitish females produced more fledglings when breeding in wooded areas, whereas reddish females produced more fledglings when breeding in wooded areas. Differently coloured females are thus adapted to different habitats. This adaptation could be due to the fact that different colourations provided camouflage in alternative habitats. But not only. Indeed, cross-fostering experiments showed that female nestlings grew wings more rapidly when both their foster and biological parents were of similar colour. It therefore seems that coloration is associated with physiological adaptations to particular environments. This result suggests that mothers should particularly produce daughters in environments that best match their own coloration. Accordingly, whiter females produced fewer daughters in territories with more arable fields. (Dreiss AN, Antoniazza S, Burri R, Fumagalli L, Sonnay C, Frey C, Goudet J & Roulin A. 2012. Local adaptation and matching habitat choice in female barn owls with respect to melanic coloration. Journal of Evolutionary Biology 25, 103-114).
Growth
- Darker reddish fathers produce longer-tailed offspring because these males pair with long-tailed females (Roulin A. 2006. Linkage disequilibrium between a melanin-based colour polymorphism and tail length in the barn owl. Biological Journal of the Linnean Society 88, 475-488).
- Paler coloured breeding males are heavier than darker reddish males. This might be a consequence of the fact that these males invest less effort in reproduction? (Roulin A. 2006. Linkage disequilibrium between a melanin-based colour polymorphism and tail length in the barn owl. Biological Journal of the Linnean Society 88, 475-488).
- Paler coloured nestling males born in our study area are more often recruited in the local breeding population than darker reddish nestling males (Roulin A & Altwegg R. 2007. Breeding rate is associated with pheomelanism in male and with eumelanism in female barn owls. Behavioral Ecology 18, 563-570). This might be the consequence of the fact that darker reddish birds disperse longer distances (Van den Brink V, Dreiss AN & Roulin A. 2012. Melanin-based colouration predicts natal dispersal in the barn owl Tyto alba. Animal Behaviour 84, 805-812; Roulin A. In press. Ring recoveries of dead birds confirm that darker pheomelanic barn owls disperse longer distances. Journal of Ornithology).
- Under relaxed rearing conditions (but not stressful conditions) nestlings grew in body mass more rapidly if their mother and father were darker reddish (Roulin A, Gasparini J, Bize P, Ritschard M & Richner H. 2008. Melanin-based colorations signal strategies to cope with poor and rich environments. Behavioral Ecology and Sociobiology 62, 507-519).
Appetite
- Whitish individuals have less appetite than reddish ones (Dreiss A, Henry I, Ruppli C, Almasi B & Roulin A. 2010. Darker eumelanic barn owls better withstand food depletion through resistance to food deprivation and low appetite. Oecologia 164, 65-71).
Dispersal behaviour
- Darker reddish males and females disperse long distances (Van den Brink V, Dreiss AN & Roulin A. 2012. Melanin-based colouration predicts natal dispersal in the barn owl Tyto alba. Animal Behaviour 84, 805-812; Roulin A. In press. Ring recoveries of dead birds confirm that darker pheomelanic barn owls disperse longer distances. Journal of Ornithology).
Social behaviour
- Darker reddish nestlings are more willing to share food with their siblings than paler nestlings. This suggests that coloration is associated with altruistic behaviours (Roulin A, Da Silva A & Ruppli CA. 2012. Dominant nestlings displaying female-like melanin coloration behave altruistically in the barn owl. Animal Behaviour 84, 1229-1236).
- In three-chick broods the oldest nestlings were likely to steal food from siblings when light reddish, whereas its youngest siblings stole food more often if darker reddish. Selfish behaviour is thus also associated with coloration (Roulin A, Da Silva A & Ruppli CA. 2012. Dominant nestlings displaying female-like melanin coloration behave altruistically in the barn owl. Animal Behaviour 84, 1229-1236).
Three key messages can be derived from the above review of studies:
- Reddish coloration is associated with physiological processes that are important at least during growth.
- Differently reddish coloured females are adapted to alternative habitats
- Reddish coloration is associated with both social and dispersal behaviours.
(Male) mate choice
An obvious issue about the adaptive function of melanin-based coloration is whether plumage traits play a role in mate choice. This aspect of animal life is not that easy to demonstrate for several reasons. First, it is not that easy to manipulate plumage traits to test experimentally whether a colour morph is more attractive to sexual partners than another morph. Second, mate choice can vary according to environmental factors. For instance, females may prefer males displaying a given plumage in one habitat (or in some years) and males showing another plumage type in another habitat (or in other years). Third, even if female mate choice is almost the rule in animals, there is still the possibility that males do not pair (or mate) randomly with respect to female coloration.
We have not only evidences that pairing with respect to the diameter of black feather spots is not random but also that males adjust their reproductive behaviour according to this female trait. 1) Assortative pairing. We found that in some years pairing with respect to spot size is assortative, random or even disassortative (unpubl. data). 2) Consistency in mating pattern. The second female of males that change partner between two successive years is similarly spotted as the previous female. This suggests that each male has a preference for females displaying spots of a specific size. 3) Differential allocation. After I cut off spots from the plumage of incubating females using scissors, I observed that their male partner reduced feeding rate which resulted in poorer offspring body condition. 4) Probability of renesting. Females for which I cut off black spots bred the following year less often than females for which I damaged their plumage but did not remove black spots.
For more information see
Roulin A. 1999. Nonrandom pairing by male barn owls Tyto alba with respect to a female plumage trait. Behavioral Ecology 10, 688-695
Roulin A & Bize P. 2007. Sexual selection in genetic colour polymorphic species: a review of experimental studies and perspectives. Journal of Ethology 25, 99-105.
Roulin A & Altwegg R. 2007. Breeding rate is associated with pheomelanism in male and with eumelanism in female barn owls. Behavioral Ecology 18, 563-570.

A barn owl family, with male on the left and female on the right. Photo S. Fabrizio.
Sexually antagonistic selection
Sexually antagonistic selection is the process by which the same phenotype is selected in opposite direction in the two sexes: males are selected in one direction and females in the other direction. For instance, in human the hip is smaller in males than in females because in the past males had to hunt (a small hip is better to run) whereas females have to give birth (it’s easier to give birth when the hip is larger).
It is quite logic to investigate whether in the barn owl plumage traits experience sexually antagonistic selection. These traits are sexually dimorphic (females are on average darker reddish and display more and larger black spots than males) but members of the two sexes can nevertheless express each phenotype. Therefore, the key question is whether to resemble a female is disadvantageous to males and whether to resemble a male is disadvantageous to females. We studied these questions in two ways, (1) by using long-term datasets collected in a free-living Swiss population and (2) by measuring the size of black spots of individuals preserved as skin specimens in Natural history Museum. The latter approach offers the unique opportunity to obtain data from several barn owl populations during a very long time period (i.e. 1816-2001).
Results from one of the most important study we wrote so far: Roulin A, Altwegg R, Jensen H, Steinsland I & Schaub M. 2010. Sex-dependent selection on an autosomal melanic female ornament promotes the evolution of sex ratio bias. Ecology Letters 13, 616-626
- In a 12-year study we show that females are strongly selected to be large-spotted, whereas males are weakly selected to be small-spotted.
- This selection was exerted on genes located on autosomes but not on those located on the Z-sex chromosome.
- Very interestingly, females have a higher survival when their father is large-spotted (and hence are female-like), whereas males have a higher survival when their mother is small-spotted.
- It is therefore not surprising that female-like males produced more daughters than sons, while male-like females produced more sons than daughters. This indicates that the disadvantage in females to display a male-like plumage (and in males a female-like plumage) may be partially compensated by adaptive sex ratio biases.
Study on skin museum specimens (Roulin A, Antoniazza S & Burri R. 2011. Spatial variation in the temporal change of male and female melanic ornamentation in the barn owl. Journal of Evolutionary Biology 24, 1403-1409).
- In seven European countries the temporal change in spot size from 1816 to 2001 was sex- and country-specific. In males, spots became smaller particularly in three countries, whereas in females the size of spots increased significantly in two countries and decreases in two others. This suggests that in the barn owl, spot size (or genetically correlated traits) is sexually antagonistically selected and that its pattern of selection. However, in contrast to our current studies in Switzerland negative selection in males appeared to be stronger than positive selection in females.

Surprising observation on the campus of the University of Lausanne: a barn owl roosting on a pole during daylight hours. Photo R. Burri.
Evolutionary stability of colour polymorphism
Maintenance of the variation in spot size. We showed on numerous occasions that females displaying larger black spots have a selective advantage. Since the expression of spots of various sizes is under strong genetic control, how can genetic variation be evolutionary stable? This is a tricky issue because we found hardly any advantage to be small-spotted in females. The studies on sexually antagonistic selection however provide a plausible solution to this key problem. Indeed, if females are selected to larger spotted, they will continue to produce large-spotted males that are counter-selected. Similarly, if males are selected to small spotted, they will continue to sire small-spotted females that are counter-selected. Sexually antagonistic selection therefore ensures that we still find small and large spotted males and females. Note that the magnitude of selection exerted in females and males can vary (independently) from year to year and also from one population to another. This is thus an interesting aspect to tackle which calls for long-term studies being performed in various populations.

A nestling barn owl displaying many large black spots and its immaculate sibling. Photo A. Roulin
Maintenance of the variation in the degree of reddishness. In the same population we can find dark reddish barn owls as well as white owls plus all possible intermediate forms. Our observations and experiments indicate that this polymorphism is maintained through local adaptation processes. In other words, dark reddish barn owls are adapted to some specific habitats (arable fields, open landscape) and whitish barn owls to habitats close to forests. The exact adaptations to these different environments are diverse. First, it appears that reddish coloration is associated with predator-prey relationships. Maybe different colorations confer camouflage in alternative habitats. Second, differently coloured barn owls perform better in different habitats. For instance, nestlings born from dark reddish mothers grow better if their nest is located in arable fields, whereas nestlings born from white mothers grow better if located near forests.

Reddish and white barn owl siblings. Photo A. Roulin.
Worldwide variation in melanin-based colour traits
So far I presented results gathered in Switzerland and few others in France and Israel. However, the barn owl is found on all continents and many islands. This offers a great opportunity to tackle the following questions:
- Where was located the ancestral population of this cosmopolitan species?
- How genetically differentiated are barn owls from different regions? How intense are genetic exchanges between different populations?
- What was the level of speciation in this cosmopolitan species?
- What could be the role of colour polymorphism in genetic differentiation between populations?
- Which ecological and climatic factors account for the evolution of different colour patterns in different populations?
- Are clines in plumage polymorphism maintained by natural selection?
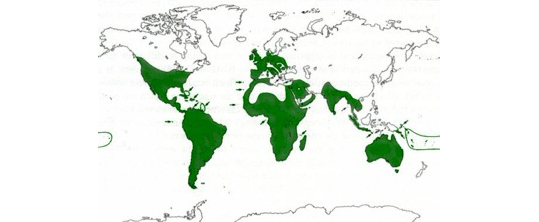
Distribution map of the barn owl. As can be seen this bird is found on all continents except in very cold regions.
Cline variation in melanin-based colour traits
Investigating phenotypic variation at a wide geographic scale is not an easy exercise because we need to sample many individuals in many different populations. However, natural history museums preserve a very large number of animals to be studied by researchers. For instance, the British Natural History Museum located at Tring as well as the American Natural History Museum located in New York have a collection of more than 1 million birds! In each of these two museums more than 1’000 barn owls are available. I therefore decided to visit museums in order to obtain data on barn owls from all continents and each single island. So far (February 2013) I visited 45 European museums, 50 US and Canadian museums, 2 African museums and 1 museum in the Middle East. This effort was worth as I measured 8’000 barn owls! Here are a number of results derived from this sample of skin specimens:
- Larger barn owl species display larger black spots. And larger species are located in regions where precipitations are more abundant.
- In the southern hemisphere barn owl species display large black spots, whereas in the northern hemisphere barn owl species located closer to the equator are larger spotted.
- Barn owl species located in the southern hemisphere are lighter reddish than species in the northern hemisphere.
- On several continents (South and North America, Europe, Asia and Africa) melanin-based coloration varies clinally. It means that birds are lighter coloured or smaller spotted in the north or south of continents.
- On islands barn owls exhibit smaller and fewer black spots and are lighter reddish than on continents.
- On islands sexual dimorphism in reddish coloration is less pronounced than on continents (i.e. on islands males tended to be as reddish as females).
- On smaller islands owls are redder pheomelanic and smaller in size than olws living on larger islands.
- Sexual dimorphism in the size of eumelanic spots is more pronounced in barn owls living on islands located further away from a continent (i.e. females displayed much larger spots than males).
- To conclude the effect of insularity was more pronounced on body size and shape than on melanic traits.
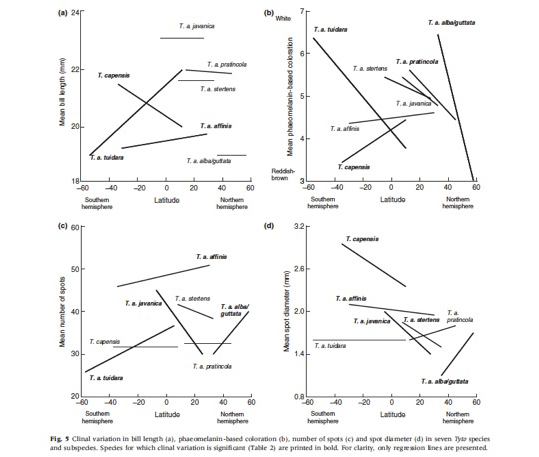
For more information see
Roulin A, Wink M & Salamin N. 2009. Selection on a eumelanic ornament is stronger in the tropics than in temperate zones in the worldwide-distributed barn owl. Journal of Evolutionary Biology 22, 345-354.
Roulin A & Salamin N. 2010. Insularity and the evolution of melanism, sexual dichromatism and body size in the worldwide-distributed barn owl. Journal of Evolutionary Biology 23, 925-934.
Cline variation on the European continent
As can be seen in the graph below, barn owls are much darker reddish in the northern compared to southern parts of Europe. This observation raises the following key questions:
- How did the cline variation emerge in the first place?
- Did colour polymorphism play a role in the emergence of this cline? We can indeed imagine that to invade northern Europe after the last Ice Age required specific adaptations such as a dark melanic plumage.
- How is this cline evolutionary stable?
So far we tackled the third question, while the first and second questions are currently being worked out. Thus, regarding the third question we asked European ornithologists to collect feathers from barn owls located throughout Europe from southern Spain/Portugal up to Denmark. This project was initiated in collaboration with Sylvain Antoniazza, Reto Burri and Jerôme Goudet who are key specialists on population genetics and quantitative genetics. Using the collected feathers we measured colour traits but also analysed microsatellites. The results show that phenotypic differentiation between populations is much more pronounced that at neutral genetic markers strongly suggesting that cline variation in coloration is very probably maintained by natural selection.
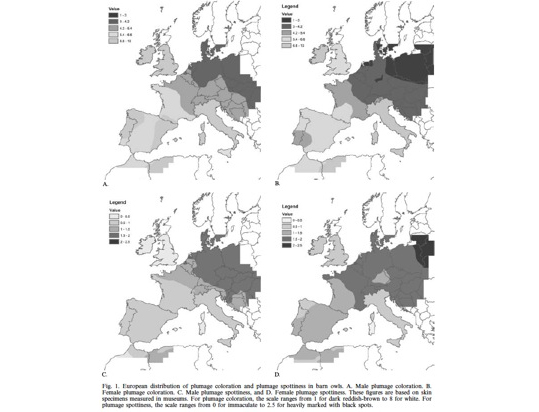
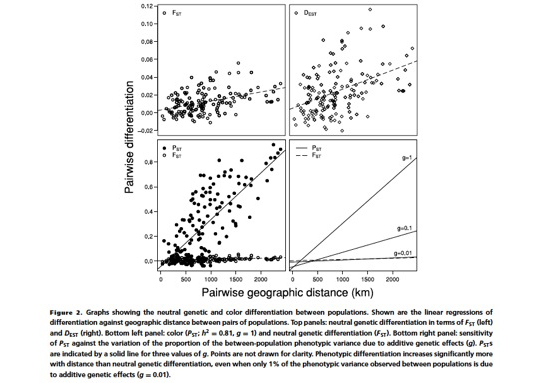
For more information see
Antoniazza S, Burri R, Fumagalli L, Goudet J & Roulin A. 2010. Local adaptation maintains clinal variation in melanin-based coloration of European barn owls (Tyto alba). Evolution 64, 1944-1954.
Roulin A. 2004. Covariation between plumage colour polymorphism and diet in the barn owl Tyto alba. Ibis 146, 509-517.
Roulin A. 2003. Geographic variation in sexually selected traits: a role for direct selection or genetic correlation? Journal of Avian Biology 34, 251-258.
Sex-specific cline variation
At least on the European continent, cline variation in coloration is maintained by natural selection. We are currently analysing samples collected throughout North America to examine whether natural selection is also responsible to maintain cline variation in melanin-based traits. Here I would like to emphasize another interesting aspect of geographic variation. Indeed, on the European continent it appeared that cline variation in the size of black spots is much more pronounced in males than females, a result that was obtained using two different methods, namely measurements taken on skin specimens preserved in museums and on feather collected by ornithologists. This observation suggests that (a) selection to heavily spotted in northern Europe and small spotted in southern Europe is much more pronounced in males than females and that (b) females are under strong selection to be heavily spotted throughout Europe. These observations call for more studies in barn owl populations located at different places in Europe.
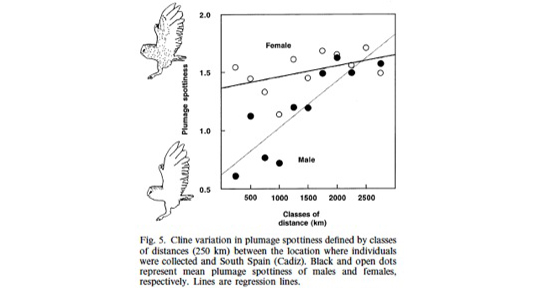
For more information see
Roulin A, Antoniazza S & Burri R. 2011. Spatial variation in the temporal change of male and female melanic ornamentation in the barn owl. Journal of Evolutionary Biology 24, 1403-1409.
Roulin A. 2003. Geographic variation in sexually selected traits: a role for direct selection or genetic correlation? Journal of Avian Biology 34, 251-258.
Mechanism
The main goal of our studies is to understand the adaptive function of melanin-based coloration and how variation in coloration is maintained within populations and why different populations evolved different melanin-based traits. As all biologists we aim at finding results that are applicable beyond the model system with which we work. During years we worked with barn owls looking at the correlates of melanin-based coloration. This was necessary to understand the implications for an owl to be reddish or whitish or to be immaculate or heavily marked with black eumelanic spots. After having shown that inter-individual variation in melanin-based coloration is mainly caused by genetic factors and only weakly influenced by the environment, we demonstrated that melanin-based coloration is associated with physiological processes, behaviour and life history traits. When we go back to the 2000s, the finding that melanin-based coloration is genetically correlated with other phenotypic attributes was unexpected and not predicted by prevailing theory. At that time, most evolutionary biologists interested in sexual selection processes considered that a colour trait can reflect quality only if this trait entails costs to its bearer. For instance, they considered that a colour trait advertises the ability to resist parasite attacks only if the trait is costly to produce or to wear. In that case, only individuals that possess the so-called good-genes can afford to produce a bright colour trait because they have the necessary genes to resist parasites and can therefore allocate resources in the production of a colourful trait. At that time I thus had difficulties to understand why a genetically inherited colour trait without any condition-dependent component could be associated with phenotypic traits including parasite resistance. This difficult was exacerbated by the fact that covariations between melanin-based coloration and other phenotypic attributes were stronger in females than males. Even editors refused to send my papers to reviewers because they could not believe the results and once one editor wrote me a letter saying that the results were biased and not biologically relevant!
What is the mechanism underlying covariation between genetically inherited melanin-based colorations and other phenotypes? When opening a textbook representing the biochemical cascade leading to the production of melanin pigments, we first notice that many genes are involved. However, there are a series of genes that appear to be very interesting due to their numerous pleiotropic effects. Genes of the melanocortin systems are indeed involved in both melanogenesis and other physiological processes.
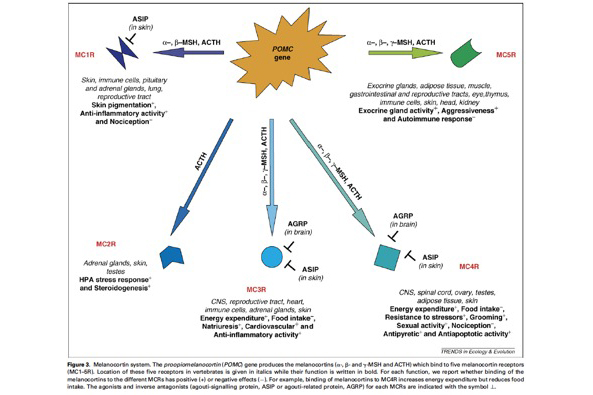
This system is very interesting for several reasons:
- A single gene (so-called POMC gene) produces several peptides that bind five different receptors (so-called melanocortin receptors) with very different physiological functions.
- We know the identity of agonists (melanocortins) and antagonists of the melanocortin receptors.
- The melanocortin system is known to be responsible for a number of genetic diseases in humans including obesity.
- This system is highly conserved in vertebrates implying that results obtained in the barn owl are either applicable to other organisms or will shed new light on the evolutionary ecology but also biomedical importance of this system.
Studying the melanocortin system is not so easy in non-model organisms. A reason is that the POMC gene is very weakly expressed implying that measuring POMC gene expression levels is not easy as well as the derived peptides (ACTH, α-MSH. We however succeeded to develop these methods and we are currently analysing the results. Thus more soon!
Group Leader
Post-Docs
Graduate Students
Master Students
Previous group members
- Laurie Ançay
- Sylvain Antoniazza
- Dr. Paul Béziers
- Dr. Reto Burri
- Morgane Calvani
- Dr. Pauline Chuarrau
- Margherita Corti
- Dr. Amélie Dreiss
- Dr. Pauline Ducouret
- Dr. Valérie Ducret
- Guillaume Dumont (co-supervision with Prof. J. Goudet)
- Guillaume Emaresi
- Susana Figueiredo Pinto
- Vanessa Formenti
- Dr. Arnaud Gaigher (en collaboration avec le groupe Salamin)
- Dr. Laura Gangoso
- Dr. Julien Gasparini
- Charlène Gémard
- Anne-Caroline Heintz
- Isabelle Henry
- Nathan Kulling
- Prof. Yossi Leshem
- Francesco Loria
- Dr. Karin Löw
- Ana Paula Machado (co-supervised by Prof. J. Goudet)
- Dr. Carolina Massa
- Eva Meyrier
- Dr. Romain Piault
- Céline Plancherel
- Charlotte Récapet
- Dr. Charlène Ruppli
- Kathleen Salin
- Dr. Luis San Jose Garcia
- Dr. Madeleine F. Scriba
- Robin Séchaud
- Vera Uva (co-supervision with Prof. L. Fumagalli)
- Dr. Valentijn Van den Brink
- Karine Vincent
- Steve Zurkinden


