KANDALAFT Lab
Our focus
We work on the clinical development of personalized cancer vaccines. The aim is to improve whole tumor lysate vaccination approaches and other personalized cancer vaccine approaches by improving the dendritic cell platform and by choosing the most immunogenic antigen with the most practical preparation to be translated rapidly to the clinic.
The other focus of our lab is to find the best setting to integrate vaccines in the standard of care treatment plan for ovarian cancer patients and to understand the immune fitness of cancer patients and correlate it with vaccination.
Our projects
- Improving whole tumor lysate vaccination approaches by enhancing the functionality of DC vaccines through genetic engineering and through comparing the different cross presentation capacity of different types of DCs.
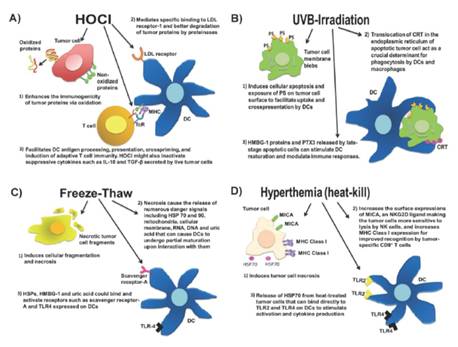
- Identifying the most effective and feasible personalized antigen for vaccination by understanding the immunogenic effect of oxidation through proteomics and immunopeptidomics in collaboration with Dr. Michal Bassani. Previous work showed that HOCl treatment of tumor cells (prior to lysis) greatly increases their immunogenicity. This work aims to identify what are the molecular and signaling mechanisms driving this improved immunogenicity.
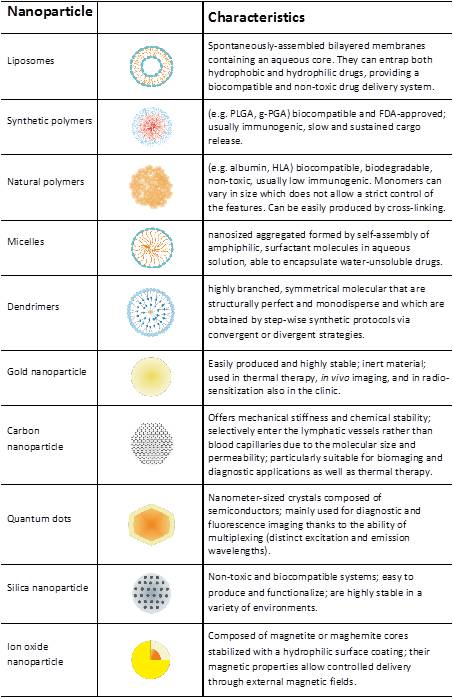
- Polymer nanomaterials for antitumor immunotherapy in collaboration with Pr Harm-Anton Klok (EPFL, the Swiss Federal Institute of Technology in Lausanne). This study focuses on the development of nanoparticles for the delivery of autologous oxidized tumor lysate to mount a cancer-specific immune response.
-
Improving vaccination strategies by integrating vaccines with the standard of care therapy in an ovarian cancer mouse model.
-
Understanding immune fitness of cancer patients and correlating it with vaccination.
KEY PUBLICATIONS

- Kandalaft L.E., Powell D. Tanyi J., Chiang, C., Torigian D., Bosch M, Levine B, June C.H., Coukos, G. Autologous Lysate-Pulsed Dendritic Cell Vaccination Followed by Adoptive Transfer of Vaccine-Primed Ex Vivo Costimulated T-cells in Recurrent Ovarian Cancer.Oncoimmunology, Jan 1;2(1):e22664.2013
- Kandalaft L.E., Tanyi J., Coukos G. Phase I Clinical Trial of Autologous OCDC Vaccine in Recurrent Ovarian Cancer. A J Transl Med. 2013 Jun 18;11(1):149
- Kandalaft L.E., Chiang, C., Tanyi J., Hagemann AR, Motz G, Sovorons N, Smith L, Montone K, Mantia-Smaldone G, Nisenbaum H, Torigian D., Czerniecki B, Powell D., Coukos G. A Dendritic Cell Vaccine Pulsed with Autologous HOCl-oxidized Ovarian Cancer Lysate Primes Effective Broad Antitumor Immunity: From Bench to Bedside.Clin Cancer Res. 2013 Jul 15.
News @ Kandalaft Lab

Results of a Phase 1 study combining personalized cancer vaccines with T-cell therapy to treat patients with ovarian cancer
Most patients with Ovarian cancer do not benefit from immune checkpoint immunotherapy and thus require alternate clinical approaches. The team had previously shown that vaccination using whole tumor-pulsed dendritic cells amplifies neoantigens recognition in ovarian cancers.
Meet all the Kandalaft Lab Members.
| Funding |
Completed:
|
|
Affiliations |
|
| Links |
|




