RUFER Lab
Our focus
We study human CD8 T-cell responses to self-(tumor) and foreign (viral) antigens with the aim to advance our knowledge of T cell-mediated protection from human disease and to improve T cell-based therapies against cancer. One focus lies in the development of adoptive cell transfer strategies using engineered T cells.
Our projects
Identifying high-avidity and high-quality individual CD8 T cells in melanoma patients
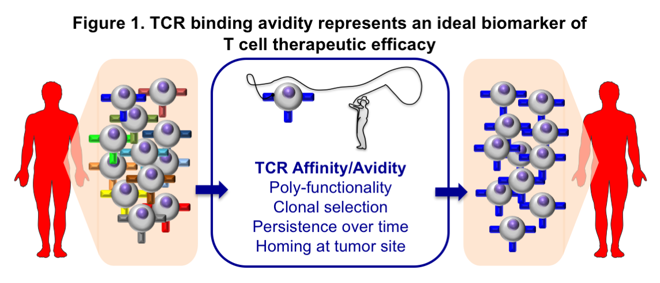
The TCR binding strength to peptide-MHC, i.e. the so-called TCR binding avidity, is viewed as a key parameter for protective T cell mediated immunity (Figure 1). Along this concept, our work offers clear evidence that the TCR binding avidity represents an ideal candidate as a biomarker of T cell functional potency (ref. 1) and therapeutic efficacy following vaccination (ref. 2). Specifically, we could address the impact of high peptide dose vaccination in promoting the selection of tumor antigen-specific CD8 T cells of enhanced functional competence (ref 2). Therapeutic vaccines also provide the unique opportunity to study the TCR clonotype selection and maintenance of vaccine-induced T cells.
Deciphering the molecular mechanisms regulating TCR affinity-increased CD8 T lymphocytes
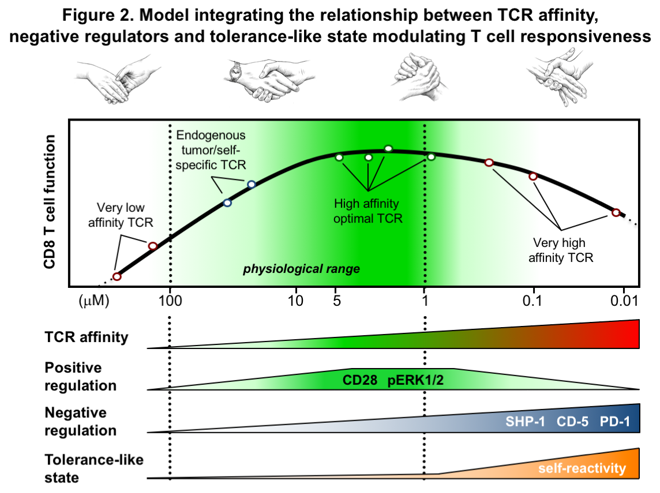
Another area of research includes the optimization of anti-tumor T cells for future adoptive cell transfer to treat cancer patients. For this purpose, our team generated a unique model of genetically engineered T-lymphocytes, by equipping them with TCRs of increased affinity against NY-ESO-1, a tumor antigen expressed in different types of cancer (Figure 2). In particular, we were able to demonstrate that the peak function of these modified T cells is calibrated by regulatory mechanisms linked to TCR affinity. Recently, we described that sustained chronic interactions between affinity-increased TCR and self-MHC can directly adjust the functional potential of engineered T cells (ref. 3). Our research underlines the importance of identifying anti-tumor TCRs capable of generating optimal function without toxicity for effective clinical application.
KEY PUBLICATIONS

- Allard M, Couturaud B, Carretero-Iglesia L, Duong MN, Schmidt J, Monnot G, Romero P, Speiser DE, Hebeisen M, and Rufer N. TCR-ligand dissociation rate is a robust and stable biomarker of CD8 T cell potency. JCI Insight. 2017
- Carretero-Iglesia, L, Couturaud B, Baumgaertner P, Schmidt J, Maby-El Hajjami H, Speiser DE, Hebeisen M, and Rufer N. High peptide dose vaccination promotes the early selection of tumor antigen-specific CD8 T cells of enhanced functional competence. Front Immunol. 10, 3016. 2020
- Duong MN, Erdes E, Hebeisen M, and Rufer N. Chronic TCR-MHC (self)-interactions limit the functional potential of TCR affinity-increased CD8 T lymphocytes. J Immunother Cancer. 7(1):284. 2019
Meet all the Rufer Lab Members.
| Funding |
|
|
Affiliations |
|







